Book Appoinment
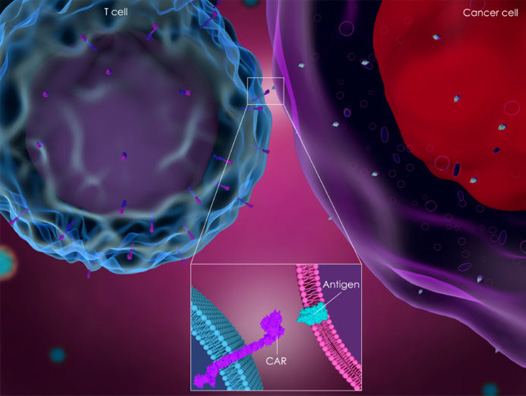
Prof V M Patil is an Car- T cell therapy expert. CAR-T cell therapy, or chimeric antigen receptor T-cell therapy, is an innovative form of immunotherapy that has shown great promise in treating certain types of cancer. I can provide you with some general information about CAR-T therapy.
Definition
CAR-T cell therapy involves genetically modifying a patient's own T cells (a type of immune cell) to express a chimeric antigen receptor (CAR) on their surface. This receptor is designed to recognize specific antigens on cancer cells.
Process
Collection: T cells are extracted from the patient's blood through a process called leukapheresis.
Modification: The T cells are then genetically engineered to express the CAR, which enables them to target cancer cells.
Expansion: The modified T cells are cultured and expanded in the laboratory to create a large population of CAR-T cells.
Infusion: The expanded CAR-T cells are infused back into the patient's bloodstream.
Targeted Antigens
CAR-T therapy is designed to target specific antigens on the surface of cancer cells. For example, Kymriah and Yescarta, two FDA-approved CAR-T therapies, target CD19, a protein found on certain B-cell cancers.
Efficacy and Challenges
- CAR-T therapy has shown remarkable success in treating some hematological malignancies, particularly certain types of leukemia and lymphoma.
- Challenges include managing cytokine release syndrome (CRS), a potentially severe immune reaction, and neurotoxicity, which can affect the nervous system.
FDA-Approved CAR-T Therapies
- Kymriah (tisagenlecleucel): Approved for certain types of leukemia and lymphoma.
- Yescarta (axicabtagene ciloleucel): Approved for certain types of non-Hodgkin lymphoma.
Ongoing Research:
Research is ongoing to expand the use of CAR-T therapy to other types of cancer and improve its effectiveness and safety profile.




